 How do those twitchy, leggy insects manage to stroll around so nonchalantly on ponds and puddles?
How do those twitchy, leggy insects manage to stroll around so nonchalantly on ponds and puddles?
Water striders, as they are known, treat the surface of a stretch of water as you or I would a wooden floor: a solid surface on which you can safely place your weight. On closer examination, it might be more accurate to liken the display to children testing out a bouncy castle. Beneath the feet, a dimple appears in the surface where the weight of the child or insect presses down on the pliable surface. The scientific question seems to be: why does a stretch of water seem to have an elastic surface when we know it’s really a liquid through and through. We’re talking ‘surface tension’ – an obscure aspect of science perhaps but one which has huge implications for the wellbeing of aquatic lifeforms, for cleaning our dirty washing and for the movement of liquids in almost all living systems.
The bouncy castle makes a good starting point. Clearly if the full weight of a child is pressing down on the rubbery floor, the latter must be pushing up equally to maintain a balance. Where does this upward push come from? This diagram offers a clue:
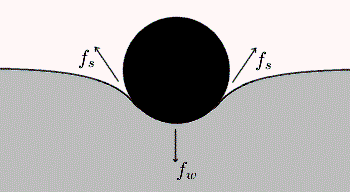 Object resting on an elastic surface
Object resting on an elastic surface
As the rubber begins to sink down under the child’s weight it gets slightly stretched. As you know from stretching any normal piece of rubber or plastic, the more you stretch it the harder it gets to extend it further. In other words, a force is building up in the material resisting your pull. This kind of force is known both scientifically and colloquially as ‘tension’. Our question now becomes what is it that causes tension? What is it about rubber or plastic – or materials in general – that resists a force attempting to pull it apart? The answer is almost self-evident once you pose the question. Whatever the material is composed of must resist being pulled apart. If it didn’t, the material wouldn’t hold together in the first place! There must be forces inside all materials that bind together the components of that material, forces that make stuff cohere. This begs the most fundamental question: what is stuff made of?
What materials are made of is one of the oldest of philosophical questions. The ancient Greek, Empedocles, proposed that substances were composed of a blends of four elements: water, air, fire and earth and this idea persisted into the middle ages. Comparable ideas were also prevalent in ancient India, Japan and the Middle East. The philosopher Democritus held that substances are made up of myriad tiny, indivisible atoms of various types and shapes.
Our contemporary understanding, developed from countless investigations in the nineteenth and twentieth centuries, combines parts of each of these ancient concepts. All materials are indeed composed of identical repeating basic units but there are countless varieties of them. The basic units of which matter is composed in normal conditions are called molecules There are molecules of familiar substances such as water, oxygen and rubber. Molecules are also the basic units of most biological substances, such as proteins, carbohydrates and DNA. What took scientists a long time to unravel is that molecules themselves can be broken down into components – they are not, in this sense, fundamental. It takes something to cause such a breakdown – combustion, a chemical reaction, or the activity of an enzyme, for example. What molecules are themselves made of we now call atoms. As an example, the water molecule H2O is made of two hydrogen atoms and one oxygen, the molecule cholesterol C₂₇H₄₆O is made of 27 carbon atoms, 46 hydrogen and 1 oxygen.
Meanwhile, back at the bouncy castle, the plastic or rubber of which it is made is itself made of molecules. These are being pulled apart when the surface is stretched by a child standing on it. Clearly, it is these molecules that are resisting the effort to stretch the surface; so, the molecules must be attracting one another. It turns out that there is indeed a force of attraction between molecules in general, at least in the solid and liquid form. Indeed it is this force that accounts for solids remaining solid and liquids holding themselves together rather than drifting off into the infinite beyond, as molecules in gases do.
This talk of molecules and forces is all very interesting, but it still doesn’t explain how the water boatman pulls off its miraculous trick. Why does the surface of water behave differently from the bulk of water beneath? Why is there tension in the surface?
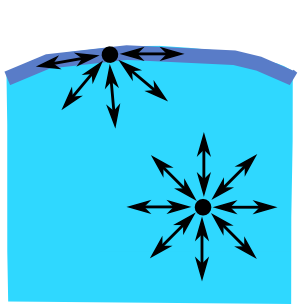
In the body of the water each H2O molecule is surrounded by other molecules on all sides. On average the pull of each molecule on its neighbours is equal on all sides – there is no net force on the molecules, no source of tension within the water. At the very surface, however, the molecules are not only in contact with their neighbouring water molecules, they are also in contact with the outside world – the air in everyday situations.
This imbalance of forces at the surface exerts a small inward pressure on molecules in the surface, forcing some into the bulk of water, leaving those on the surface a little more thinly spread. As a result the H2O molecules near the surface are slightly further apart from one another as shown in the yellow zone in the diagram which represents the boundary between a liquid (Ll) and vapour (Lv)
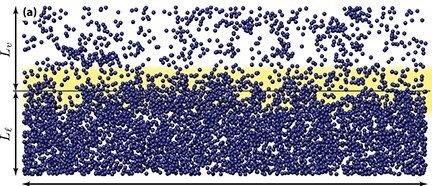 H2O molecules in water, air and the boundary layer in between
H2O molecules in water, air and the boundary layer in between
(From Europhysics Letters, 2015, Volume 109, Number 4 with kind permission of the publisher)

But, as we have just seen, there are gentle forces of attraction between neighbouring molecules. So, when molecules are pulled further apart from one another, a tension forces arises, trying to pull them back closer to one another. It’s rather like the force in the spring of a chest expander when you stretch it, the spring tries pull back to the slack position.
Tension in a stretched spring
So, like a child on a bouncy castle, the water boatman is walking on a stretchable surface. The tension in the surface causes dimples to form under its feet. The upward part of the surface tension balances the insect’s weight, as shown by the force labelled Fs in the diagram near the top of this blog.

Click here for a bit of fun watching some water boatmen at play on a sunny day on the river Lea.
As an aside, the surface tension of water is relatively high compared to other kinds of liquid. This is due to an extra kind of bond that exists between molecules of H2O, called a hydrogen bond. It’s these extra strength bonds that make small quantities of water curl up into droplets, as they do dripping from a leaky tap, rather than spreading out. Paradoxically this high level of tension in the surface of water also means it doesn’t always wet other surfaces very well. You can see this sometimes when you’re doing the laundry; because of the surface tension, water molecules don’t always penetrate right into the fibres of cloth. This can mean pockets of dirt that are attached to the fibres are not always dissolved away.
This is why we add soap or detergent to water; it helps by reducing the surface tension, which enables more dirt to get dissolved.
The molecules of soaps and detergents are long thin molecules with one end that sits easily in water (red balls in the diagram) and the other more easily in grease or oil (squiggly lines). These molecules tend to congregate at the surface of a lump of grease or oil with the oily end sticking into the grease or oil and the water-loving end sticking out into the water. This way the greasy material can be lifted off textiles or dishes and float off into the water to be rinsed away.
 The molecules of soaps and detergents are long thin molecules with one end that sits easily in water (red balls in the diagram) and the other more easily in grease or oil (squiggly lines). These molecules tend to congregate at the surface of a lump of grease or oil with the oily end sticking into the grease or oil and the water-loving end sticking out into the water. This way the greasy material can be lifted off textiles or dishes and float off into the water to be rinsed away.
The molecules of soaps and detergents are long thin molecules with one end that sits easily in water (red balls in the diagram) and the other more easily in grease or oil (squiggly lines). These molecules tend to congregate at the surface of a lump of grease or oil with the oily end sticking into the grease or oil and the water-loving end sticking out into the water. This way the greasy material can be lifted off textiles or dishes and float off into the water to be rinsed away.
So, let’s marvel at the extraordinary path of evolution that has brought us the water boatman. It is so well adapted to the watery ground on which it walks that, for it, water isn’t even wet.
© Andrew Morris 11th April 2019 c
