Abstract
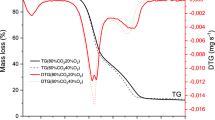
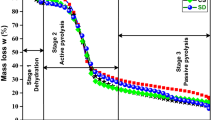
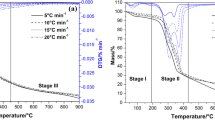
Biomass-derived humins produced in the biorefining of biomass represent an attractive feedstock for thermochemical processes. This work examines the purification and characterization of humins derived from sugarcane bagasse and rice husks (H-SCB and H-RH, respectively), followed by the kinetic and thermodynamic analysis of its pyrolysis. Pyrolysis was assessed via thermogravimetric analysis, and a global reaction model was adopted to address pyrolysis kinetics. To boost the quality of fit between the kinetic model and thermoanalytical data, the analyses are based on Vyazovkin's method. The activation energy of H-SCB increased from 166.09 to 329.76 kJ mol−1. In contrast, the activation energy of H-RH decreased from 163.31 to 84.99 kJ mol−1. According to the results of the generalized master-plot approach, the governing reaction mechanism shifted among order-based models, nucleation, and diffusion-controlled particle mechanisms. Thermodynamic properties showed that the process is endothermic, with the thermal decomposition of H-SCB being more reactive (ΔSaverage = -0.004 kJ mol−1 K−1) compared to H-RH (ΔSaverage = -0.05 kJ mol−1 K−1). Also, the heat absorbed helps the humins to achieve a more ordered state close to a conversion of 0.50. Furthermore, a difference of about 7 kJ mol−1 between the enthalpy of the reaction and the average activation energy indicates the formation of favorable product with humins’ considerable bioenergy potential. These findings are the first reported data on the forecast kinetic curves and pyrolysis mechanism of biorefinery-derived humins, and these results will enable process design for the thermochemical conversion of these emerging materials to produce energy and other products.






Similar content being viewed by others
Data availability
E-supplementary information for this work can be found in e-version of this paper online.
References
Lopes ES, Leal Silva JF, Rivera EC, Gomes AP, Lopes MS, Maciel Filho R, Tovar LP (2020) Challenges to levulinic acid and humins valuation in the sugarcane bagasse biorefinery concept. Bioenerg Res 13(3):757–774. https://doi.org/10.1007/s12155-020-10124-9
Lopes ES, Gariboti JCJ, Feistel L, Rivera EC, Maciel Filho R, Tovar LP (2020) Acid Hydrolysis-based Sugarcane Bagasse Biorefining for Levulinic Acid Production: Dynamic Mechanistic Modeling Under Varying Operating Conditions. Chem Eng Trans 80:217–222. https://doi.org/10.3303/CET2080037
Lopes ES, Rivera EC, Gariboti JCdJ, Feistel LHZ, Dutra JV, Maciel Filho R, Tovar LP (2020) Kinetic insights into the lignocellulosic biomass-based levulinic acid production by a mechanistic model. Cellulose 27(10):5641–5663. https://doi.org/10.1007/s10570-020-03183-w
Fleig OP, Lopes ES, Rivera EC, Maciel Filho R, Tovar LP (2018) Concept of rice husk biorefining for levulinic acid production integrating three steps: Multi-response optimization, new perceptions and limitations. Process Biochem 65:146–156. https://doi.org/10.1016/j.procbio.2017.11.015
Leal Silva JF, Mariano AP, Maciel Filho R (2021) Less severe reaction conditions to produce levulinic acid with reduced humins formation at the expense of lower biomass conversion: Is it economically feasible? Fuel Commun 9:100029. https://doi.org/10.1016/j.jfueco.2021.100029
Kang S, Fu J, Zhang G (2018) From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew Sustain Energy Rev 94:340–362. https://doi.org/10.1016/j.rser.2018.06.016
Shao Y, Lu W, Meng Y, Zhou D, Zhou Y, Shen D, Long Y (2021) The formation of 5-hydroxymethylfurfural and hydrochar during the valorization of biomass using a microwave hydrothermal method. Sci Total Environ 755:142499. https://doi.org/10.1016/j.scitotenv.2020.142499
Macedo MGS, Gariboti JCJ, Lopes ES, Gomes EL, Felisbino RF, Ardila YC, Tovar LP (2021) From sugarcane bagasse-derived humins towards porous materials with high-energy potential. 43rd Symposium on Biomaterials, Fuels and Chemicals, Virtual Conference
Muralidhara A, Tosi P, Mija A, Sbirrazzuoli N, Len C, Engelen V, de Jong E, Marlair G (2018) Insights on thermal and fire hazards of humins in support of their sustainable use in advanced biorefineries. ACS Sustainable Chem Eng 6(12):16692–16701. https://doi.org/10.1021/acssuschemeng.8b03971
Higgins LJR, Brown AP, Harrington JP, Ross AB, Kaulich B, Mishra B (2020) Evidence for a core-shell structure of hydrothermal carbon. Carbon 161:423–431. https://doi.org/10.1016/j.carbon.2020.01.060
Tosi P, van Klink GPM, Celzard A, Fierro V, Vincent L, de Jong E, Mija A (2018) Auto-crosslinked rigid foams derived from biorefinery byproducts. Chemsuschem 11(16):2797–2809. https://doi.org/10.1002/cssc.201800778
Dinu R, Mija A (2019) Cross-linked polyfuran networks with elastomeric behaviour based on humins biorefinery by-products. Green Chem 21(23):6277–6289. https://doi.org/10.1039/C9GC01813A
Yang J, Niu X, Wu H, Zhang H, Ao Z, Zhang S (2020) Valorization of humin as a glucose derivative to fabricate a porous carbon catalyst for esterification and hydroxyalkylation/alkylation. J Waste Manag 103:407–415. https://doi.org/10.1016/j.wasman.2020.01.004
Dinu R, Montes S, Orange F, Mija A (2021) Reprocessable humins thermosets and composites. Comp Sci Tech 207:108655. https://doi.org/10.1016/j.compscitech.2021.108655
Sun J, Cheng H, Zhang Y, Zhang Y, Lan X, Zhang Y, Xia Q, Ding D (2021) Catalytic hydrotreatment of humins into cyclic hydrocarbons over solid acid supported metal catalysts in cyclohexane. J Energy Chem 53:329–339. https://doi.org/10.1016/j.jechem.2020.05.034
Morone A, Apte M, Pandey RA (2015) Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew Sustain Energy Rev 51:548–565. https://doi.org/10.1016/j.rser.2015.06.032
van Zandvoort I, Wang Y, Rasrendra CB, van Eck ERH, Bruijnincx PCA, Heeres HJ, Weckhuysen BM (2013) Formation, molecular structure, and morphology of humins in biomass conversion: influence of feedstock and processing conditions. Chemsuschem 6(9):1745–1758. https://doi.org/10.1002/cssc.201300332
Magdziarz A, Wilk M, Wądrzyk M (2020) Pyrolysis of hydrochar derived from biomass – Experimental investigation. Fuel 267:117246. https://doi.org/10.1016/j.fuel.2020.117246
Agarwal S, van Es D, Heeres HJ (2017) Catalytic pyrolysis of recalcitrant, insoluble humin byproducts from C6 sugar biorefineries. J Anal Appl Pyrolysis 123:134–143. https://doi.org/10.1016/j.jaap.2016.12.014
Mija A, van der Waal JC, Pin J-M, Guigo N, de Jong E (2017) Humins as promising material for producing sustainable carbohydrate-derived building materials. Constr Build Mater 139:594–601. https://doi.org/10.1016/j.conbuildmat.2016.11.019
Wang S, Lin H, Zhao Y, Chen J, Zhou J (2016) Structural characterization and pyrolysis behavior of humin by-products from the acid-catalyzed conversion of C6 and C5 carbohydrates. J Anal Appl Pyrolysis 118:259–266. https://doi.org/10.1016/j.jaap.2016.02.009
Cortés AM, Bridgwater AV (2015) Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process Technol 138:184–193. https://doi.org/10.1016/j.fuproc.2015.05.013
Sbirrazzuoli N (2020) Interpretation and physical meaning of kinetic parameters obtained from isoconversional kinetic analysis of polymers. Polymers (Basel) 12(6):1280. https://doi.org/10.3390/polym12061280
Sangregorio A, Guigo N, Jong ED, Sbirrazzuoli N (2019) Kinetics and chemorheological analysis of cross-linking reactions in humins. Polymers (Basel) 11(11):1804. https://doi.org/10.3390/polym11111804
Song G, Novotny E, Simpson A, Clapp CE, Hayes MHB (2008) Sequential exhaustive extraction of a Mollisol soil, and characterizations of humic components, including humin, by solid and solution state NMR. Eur J Soil Sci 59:505–516. https://doi.org/10.1111/j.1365-2389.2007.01006.x
American Society for Testing and Materials (2017) ASTM D3173 / D3173M–17a, Standard test method for moisture in the analysis sample of coal and coke. American Society for Testing and Materials, West Conshohocken. https://doi.org/10.1520/D3173_D3173M-17A
American Society for Testing and Materials (2018) ASTM D3174–02, Standard test method for ash in the analysis sample of coal and coke from coal. American Society for Testing and Materials, West Conshohocken. https://doi.org/10.1520/D3174-12R18
American Society for Testing and Materials (2020) ASTM D3175–20, Standard test method for volatile matter in the analysis sample of coal and coke. American Society for Testing and Materials, West Conshohocken. https://doi.org/10.1520/D3175-20
Nhuchhen DR (2016) Prediction of carbon, hydrogen, and oxygen compositions of raw and torrefied biomass using proximate analysis. Fuel 180:348–356. https://doi.org/10.1016/j.fuel.2016.04.058
Trache D, Abdelaziz A, Siouani B (2016) A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J Therm Anal Calorim. https://doi.org/10.1007/s10973-016-5962-0
Flynn JH, Wall LA (1966) General treatment of the thermogravimetry of polymers. J Res Nat Bur Standards, Section A 70A(6):487–523. https://doi.org/10.6028/jres.070A.043
Starink MJ (2003) The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta 404(1):163–176. https://doi.org/10.1016/S0040-6031(03)00144-8
Vyazovkin S, Dollimore D (1996) Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Comp Sci 36(1):42–45. https://doi.org/10.1021/ci950062m
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520(1):1–19. https://doi.org/10.1016/j.tca.2011.03.034
Pérez-Maqueda LA, Criado JM (2000) The accuracy of Senum and Yang’s approximations to the Arrhenius integral. J Therm Anal Calorim 60(3):909–915. https://doi.org/10.1023/A:1010115926340
Criado JM (1978) Kinetic analysis of DTG data from master curves. Thermochim Acta 24(1):186–189. https://doi.org/10.1016/0040-6031(78)85151-X
Eyring H (1935) The activated complex in chemical reactions. J Chem Phys 3:107. https://doi.org/10.1063/1.1749604
Sumerskii IV, Krutov SM, Zarubin MY (2010) Humin-like substances formed under the conditions of industrial hydrolysis of wood. Russ J Appl Chem 83(2):320–327. https://doi.org/10.1134/S1070427210020266
Cheng Z, Everhart JL, Tsilomelekis G, Nikolakis V, Saha B, Vlachos DG (2018) Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem 20(5):997–1006. https://doi.org/10.1039/C7GC03054A
Hoang TMC, van Eck ERH, Bula WP, Gardeniers JGE, Lefferts L, Seshan K (2015) Humin based by-products from biomass processing as a potential carbonaceous source for synthesis gas production. Green Chem 17(2):959–972. https://doi.org/10.1039/C4GC01324G
Björnerbäck F, Hedin N (2019) Microporous humins prepared from sugars and bio-based polymers in concentrated sulfuric acid. ACS Sustainable Chem Eng 7(1):1018–1027. https://doi.org/10.1021/acssuschemeng.8b04658
Alves JLF, Da Silva JCG, da Silva Filho VF, Alves RF, de Araujo Galdino WV, Andersen SLF, De Sena RF (2019) Determination of the bioenergy potential of brazilian pine-fruit shell via pyrolysis kinetics, thermodynamic study, and evolved gas analysis. Bioenerg Res 12(1):168–183. https://doi.org/10.1007/s12155-019-9964-1
Zhang S, Pi M, Su Y, Xu D, Xiong Y, Zhang H (2020) Physiochemical properties and pyrolysis behavior evaluations of hydrochar from co-hydrothermal treatment of rice straw and sewage sludge. Biomass Bioenergy 140:105664. https://doi.org/10.1016/j.biombioe.2020.105664
Shahid A, Ishfaq M, Ahmad MS, Malik S, Farooq M, Hui Z, Batawi AH, Shafi ME, Aloqbi AA, Gull M, Mehmood MA (2019) Bioenergy potential of the residual microalgal biomass produced in city wastewater assessed through pyrolysis, kinetics and thermodynamics study to design algal biorefinery. Bioresour Technol 289:121701. https://doi.org/10.1016/j.biortech.2019.121701
Radojević M, Janković B, Jovanović V, Stojiljković D, Manić N (2018) Comparative pyrolysis kinetics of various biomasses based on model-free and DAEM approaches improved with numerical optimization procedure. PLoS ONE 13(10):e0206657. https://doi.org/10.1371/journal.pone.0206657
Olszewski MP, Arauzo PJ, Maziarka PA, Ronsse F, Kruse A (2019) Pyrolysis kinetics of hydrochars produced from brewer’s spent grains. Catalysts 9(7):625. https://doi.org/10.3390/catal9070625
Sobek S, Werle S (2020) Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel 261:116459. https://doi.org/10.1016/j.fuel.2019.116459
Bartocci P, Tschentscher R, Stensrød RE, Barbanera M, Fantozzi F (2019) Kinetic analysis of digestate slow pyrolysis with the application of the master-plots method and independent parallel reactions scheme. Molecules 24(9):1657. https://doi.org/10.3390/molecules24091657
Vasudev V, Ku X, Lin J (2020) Pyrolysis of algal biomass: Determination of the kinetic triplet and thermodynamic analysis. Bioresour Technol 317:124007. https://doi.org/10.1016/j.biortech.2020.124007
Sbirrazzuoli N (2019) Advanced isoconversional kinetic analysis for the elucidation of complex reaction mechanisms: a new method for the identification of rate-limiting steps. Molecules 24(9):1683. https://doi.org/10.3390/molecules24091683
Acknowledgements
The authors would like to thank Centro de Equipamentos e Serviços Multiusuários—CESM (Institute of Environmental, Chemical and Pharmaceutical Sciences at Federal University of São Paulo) for the support with TGA and the Laboratory of Recycling, Waste Treatment and Extraction—LAREX (Department of Chemical Engineering, Escola Politécnica of the University of São Paulo) for the support with TG-QMS.
Funding
This work was supported by National Council for Scientific and Technological Development—CNPq [grants 408149/2018–3 and 162373/2020–1] and São Paulo Research Foundation—FAPESP [grants 2020/11347–5, 2015/17592–3, and 2015/20630–4].
Author information
Authors and Affiliations
Contributions
Julio César de Jesus Gariboti was involved in conceptualization, methodology (kinetic analysis), validation, formal analysis, data curation, writing - original draft; Marina Gontijo Souza Macedo helped in conceptualization, methodology (purification and physicochemical characteristics), data curation, writing - original draft; Vinícius Matheus Silva Macedo contributed to methodology (purification and physicochemical characteristics), Yesid Javier Rueda was involved in conceptualization, validation (kinetic analysis), writing- reviewing and editing; Emília Savioli Lopes helped in methodology (production of agro-industrial acid hydrolysis residue); Jonathan Tenorio Vinhal contributed to methodology (TG-QMS analysis), validation, writing - original draft, Eliezer Ladeia Gomes was involved in conceptualization, validation, writing - original draft, supervision; Jorge Alberto Soares Tenório helped in validation, writing - reviewing and editing; Romilda Fernandez Felisbino contributed to validation, writing - original draft, supervision; Melina Savioli Lopes was involved in conceptualization, validation (thermodynamic analysis), writing- reviewing and editing; Laura Plazas Tovar helped in conceptualization, validation, writing - original draft, writing - reviewing and editing, resources, visualization, supervision, funding acquisition and project administration
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Registers CEP N° 6389190320, 2804210420, and 5800140620 by Comitê de Ética em Pesquisa da Universidade Federal de São Paulo/Hospital São Paulo.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Jesus Gariboti, J.C., Macedo, M.G.S., Macedo, V.M.S. et al. Elucidating the thermal decomposition mechanism and pyrolysis characteristics of biorefinery-derived humins from sugarcane bagasse and rice husk. Bioenerg. Res. 15, 2026–2044 (2022). https://doi.org/10.1007/s12155-022-10412-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10412-6