Study on the Antinociceptive Activity and Mechanism of Action of Isolated Saponins from Siolmatra brasiliensis (Cogn.) Baill
Abstract
:1. Introduction
2. Results
2.1. Assessment of Side Effects and Toxicity
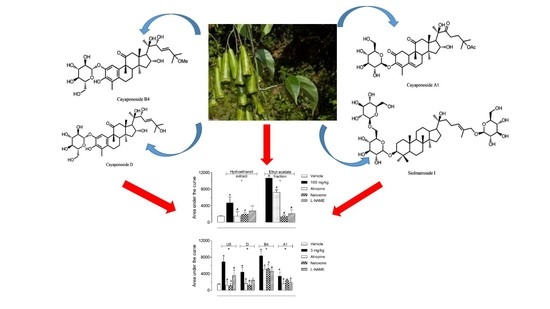
2.2. Antinociceptive Effect of HE, EtOAc, SI, D, B4, and A1 in the Hot Plate Test
2.3. Investigation of the Mechanism of Action of EtOAc, SI, D, B4, and A1 in the Hot Plate Model
2.4. Antinociceptive Effect of HE, EtOAc, SI, D, B4, and A1 in the Capsaicin- or Glutamate-Induced Nociception
3. Discussion
4. Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Animals
4.4. Extracts, Isolated Substances, and Control Drugs’ Administration
4.5. Drugs and Reagents
4.6. Acute Toxicity
4.7. Hot Plate Test
4.8. Analysis of the Mechanisms of Action of Hydroethanol Extract (HE), Ethyl Acetate Fraction (EtOAc), and its Isolated Saponins
4.9. Capsaicin- and Glutamate-Induced Nociception
4.10. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lima, A.P.; Barbosa, C.E.S.; Pereira, F.C.; Vilanova-Costa, C.A.S.T.; Ribeiro, A.S.B.B.; Silva, H.D.; Azavedo, N.R.; Gomes-Klein, V.R.; Silveira-Lacerda, E.P. Siolmatra brasiliensis (Cogn.) Baill., Cucurbitaceae, acute toxicity in mice. Rev. Bras. Farm. 2010, 20, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Pott, A.; Pott, V.J. Plantas do Pantanal; Embrapa: Brasília, Brazil, 1994. [Google Scholar]
- Santos, C.H.C.; Borges, I.P.; Silva, V.C.; Sousa, P.T.; Kawashita, N.H.; Baviera, A.M.; Carvalho, M.G. A new dammarane saponin and other triterpenoids from Siolmatra brasiliensis and evaluation of the antidiabetic activity of its extract. Pharm. Biol. 2016, 54, 1–9. [Google Scholar]
- Mogil, J.S.; Adhikari, S.M. Hot and cold nociception are genetically correlated. J. Neurosci. 1999, 19, RC25. [Google Scholar] [CrossRef]
- Ijeoma, U.F.; Aderonke, S.O.; Ogbonna, O.; Augustina, M.A.; Ifeyinwa, C.N. Antinociceptive and anti-inflammatory activities of crude extracts of Ipomoea involucrata leaves in mice and rats. Asian Pac. J. Trop. Med. 2011, 4, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Negri, G.; Mattei, R.; Mendes, F.R. Antinociceptive activity of the HPLC- and MS-standardized hydroethanolic extract of Pterodon emarginatus vogel leaves. Phytomedicine 2014, 21, 1062–1069. [Google Scholar] [CrossRef] [Green Version]
- Adzu, B.; Amizan, M.B.; Okhale, S.E. Evaluation of antinociceptive and anti-inflammatory activities of standardised root bark extract of Xeromphis nilotica. J. Ethnopharmacol. 2014, 158, 271–275. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Wess, J.; Duttaroy, A.; Gomeza, J.; Zhang, W.; Yamada, M.; Felder, C.C.; Bernardini, N.; Reeh, P.W. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: A review. Life Sci. 2003, 72, 2047–2954. [Google Scholar] [CrossRef]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef]
- Khana, H.; Saeeda, M.; Gilanib, A.; Khanc, M.A.; Dard, A.; Khana, I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J. Ethnopharmacol. 2010, 127, 521–527. [Google Scholar] [CrossRef]
- Binotti, R.S.; Melo, A.M.T.; Oliveira, C.H.; De Nucci, G. Pimenta-vermelha (Capsicum fructescens—SOLANACEAE). J. Bras. Fitomed. 2003, 1, 6–11. [Google Scholar]
- Jancso, G. Selective degeneration of chemo sensitive primary sensory neurons induced by capsaicin: Glial changes. Cell Tissue Res. 1978, 195, 145–152. [Google Scholar] [CrossRef]
- Palazzo, E.; De Novellis, V.; Marabese, I.; Cuomo, D.; Rossi, F.; Berrino, L.; Rossi, F.; Maione, S. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur. J. Pharm. 2002, 439, 69–75. [Google Scholar] [CrossRef]
- Afrah, A.W.; Stiller, C.O.; Olgart, L.; Brodin, E.; Gustafsson, H. Involvement of spinal N-methyl-D-aspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci. Lett. 2001, 316, 83–86. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Kim, M.S.; Usachev, Y.M. Mechanisms of prolonged presynaptic Ca2þ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J. Neurosci. 2008, 28, 5295–5311. [Google Scholar] [CrossRef] [Green Version]
- Himeno, E.; Nagao, T.; Honda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Structures of new non-aromatized nor-cucurbitacin glucosides in the roots of Cayaponia tayuya. Chem. Pharm. Bull. 1993, 41, 986–988. [Google Scholar] [CrossRef] [Green Version]
- Himeno, E.; Nagao, T.; Honda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Studies on the constituents of Cayaponia tayuya (Vell.) Cogn. I. Structures of Cayaponosides, new 29-nor-1,2,3,4,5,10-hexadehydrocucurbitacins glucosides. Chem. Pharm. Bull. 1994, 42, 2295–2300. [Google Scholar] [CrossRef] [Green Version]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Sahley, T.L.; Berntson, G.G. Antinociceptive effects of central and systemic administration of nicotine in the rat. Psychopharmacology 1979, 65, 279–283. [Google Scholar] [CrossRef]
- Matheus, M.E.; Berrondo, L.F.; Vieitas, E.C.; Menezes, F.S.; Fernandes, P.D. Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J. Ethnopharmacol. 2005, 102, 377–381. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.V.; Meyre-Silva, C.; Malheiros, A.; Muller, L.A.; Cani, G.S.; Santos, A.R.; Yunes, R.A.; Calixto, J.B. Antinociceptive properties of mixture of alpha-amyrin and beta-amyrintriterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J. Pharm. Exp. 2005, 313, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabarelli, Z.; Berlese, D.B.; Sauzem, P.D.; Rubin, M.A.; Missio, T.P.; Teixeira, M.V.; Sinhorin, A.P.; Martins, M.A.P.; Zanatta, N.; Bonacorso, H.G.; et al. Antinociceptive effect to novel pyrazolines in mice. Braz. J. Med. Biol. Res. 2004, 37, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, M.M.G.; Bessa, S.O.; Fingolo, C.E.; Kuster, R.M.; Matheus, M.E.; Menezes, F.S.; Fernandes, P.D. Antinociceptive activity of fractions from Couroupita guianensis Aubl. leaves. J. Ethnopharmacol. 2010, 127, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Katsumata, K.; Tanno, K.; Sakurada, S.; Kisara, K. The Capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology 1992, 31, 1279–1285. [Google Scholar] [CrossRef]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds hydroethanol extract (HE), ethyl acetate fraction (EtOAc), siolmatroside I (SI), cayaponoside D (D), cayaponoside B4 (B4) and cayaponoside A1 (A1) are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorno, T.B.S.; Santos, C.H.C.d.; Carvalho, M.G.d.; Silva, V.C.d.; Sousa, P.T.d., Jr.; Fernandes, P.D.; Boylan, F. Study on the Antinociceptive Activity and Mechanism of Action of Isolated Saponins from Siolmatra brasiliensis (Cogn.) Baill. Molecules 2019, 24, 4584. https://doi.org/10.3390/molecules24244584
Giorno TBS, Santos CHCd, Carvalho MGd, Silva VCd, Sousa PTd Jr., Fernandes PD, Boylan F. Study on the Antinociceptive Activity and Mechanism of Action of Isolated Saponins from Siolmatra brasiliensis (Cogn.) Baill. Molecules. 2019; 24(24):4584. https://doi.org/10.3390/molecules24244584
Chicago/Turabian StyleGiorno, Thais Biondino Sardella, Carlos Henrique Corrêa dos Santos, Mario Geraldo de Carvalho, Virgínia Cláudia da Silva, Paulo Teixeira de Sousa, Jr., Patricia Dias Fernandes, and Fabio Boylan. 2019. "Study on the Antinociceptive Activity and Mechanism of Action of Isolated Saponins from Siolmatra brasiliensis (Cogn.) Baill" Molecules 24, no. 24: 4584. https://doi.org/10.3390/molecules24244584