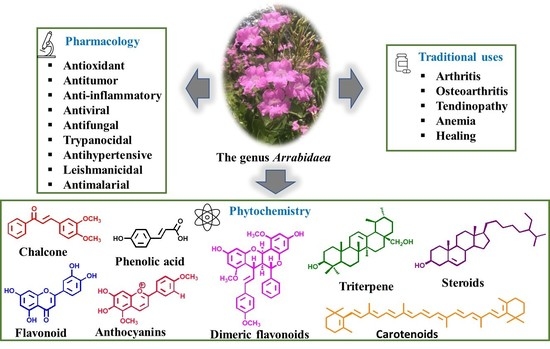
A Review of the Phytochemistry and Pharmacological Properties of the Genus Arrabidaea
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Main Bio-Pharmacological Properties and Chemical Composition of the Species from the Genus Arrabidaea
3.1.1. A. samydoides
Main Chemical Composition of A. samydoides
Bio-Pharmacological Properties of A. samydoides
3.1.2. A. pulchra
Main Chemical Composition of A. pulchra
Bio-Pharmacological Properties of A. pulchra
3.1.3. A. triplinervia
Main Chemical Composition of A. triplinervia
Bio-Pharmacological Properties of A. triplinervia
3.1.4. A. bilabiata
Main Chemical Composition of A. bilabiata
Bio-Pharmacological Properties of A. bilabiata
3.1.5. A. brachypoda
Main Chemical Composition of A. brachypoda
Bio-Pharmacological Properties of A. brachypoda
3.1.6. A. chica
Main Chemical Composition of A. chica
Bio-Pharmacological Properties of A. chica
3.1.7. A. patellifera
Main Chemical Composition of A. patellifera
Bio-Pharmacological Properties of A. patellifera
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauletti, P.M.; Bolzani, V.S.; Young, M.C.M. Constituintes químicos de Arrabidaea samydoides (Bignoniaceae). Quim. Nova 2003, 26, 641–643. [Google Scholar] [CrossRef] [Green Version]
- Bramara, B.V.B.; Vasavi, H.S.; Sudeep, H.V.; Shyam Prasad, K. Hydroalcoholic extract from Lepidium meyenii (Black Maca) root exerts wound healing activity in Streptozotocin-induced diabetic rats. Wound Med. 2017, 19, 75–81. [Google Scholar] [CrossRef]
- Silva, M.M.; Queiroz, L.P. A família Bignoniaceae na região de Catolés, Chapada Diamantina, Bahia, Brasil. Sitientibus Série Ciências Biológicas 2003, 3, 3–21. [Google Scholar]
- Sandwith, N.Y. Contributions to the Flora of Tropical America: LVII. Studies in Bignoniaceae, XX. Kew Bull. 1954, 9, 597–614. [Google Scholar] [CrossRef]
- Sandwith, N.Y. Contributions to the Flora of Tropical America: LXXVI. Notes on Bignoniaceae: XXIX: Arrabidaea in Martius’s “Flora brasiliensis” and Subsequently. Kew Bull. 1968, 22, 403–420. [Google Scholar] [CrossRef]
- Gentry, A.H. Generic delimitations of Central American Bignoniaceae. Brittonia 1973, 25, 226–242. [Google Scholar] [CrossRef]
- Alcerito, T.; Barbo, F.E.; Negri, G.; Santos, D.Y.A.C.; Meda, C.I.; Young, M.C.M.; Chávez, D.; Blatt, C.T.T. Foliar epicuticular wax of Arrabidaea brachypoda: Flavonoids and antifungal activity. Biochem. Syst. Ecol. 2002, 30, 677–683. [Google Scholar] [CrossRef]
- Zorn, B.; Garcı, A.J.; Castro, V.; Murillo, R.; Mora, G.; Melfort, I. 3-Desoxyanthocyanidins from Arrabidaea chica. Phytochemistry 2001, 56, 831–835. [Google Scholar] [CrossRef]
- Neto, G.G.; Morais, R.G. Recursos medicinais de espécies do Cerrado de Mato Grosso: Um estudo bibliográfico. Acta Bot. Bras. 2003, 17, 561–584. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Castro-Gamboa, I.; Silva, D.H.S.; Young, M.C.M.; Tomazela, D.M.; Eberlin, M.N.; Bolzani, V.S. New Antioxidant C-Glucosylxanthones from the Stems of Arrabidaea samydoides. J. Nat. Prod. 2003, 66, 1384–1387. [Google Scholar] [CrossRef]
- Rocha, C.Q.; De-Faria, F.M.; Marcourt, L.; Ebrahimi, S.N.; Kitano, B.T.; Ghilardi, A.F.; Luiz Ferreira, A.; de Almeida, A.C.A.; Dunder, R.J.; Souza-Brito, A.R.M.; et al. Gastroprotective effects of hydroethanolic root extract of Arrabidaea brachypoda: Evidences of cytoprotection and isolation of unusual glycosylated polyphenols. Phytochemistry 2017, 135, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, A.; Rocha, V.P.C.; Bastos, T.M.; Marcourt, L.; Morin, H.; Rocha, C.Q.; Grimaldi, G.B.; de Sousa, K.A.F.; Borges, J.N.; Rivara-Minten, E.; et al. Production of highly active antiparasitic compounds from the controlled halogenation of the Arrabidaea brachypoda crude plant extract. J. Nat. Prod. 2020, 83, 2631–2640. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Pessôa, A.C.M.; Carvalho, N.S. The Brazilian Amazon deforestation rate in 2020 is the greatest of the decade. Nat. Ecol. Evol. 2021, 5, 144–145. [Google Scholar] [CrossRef]
- Brandão, G.C.; Kroon, E.G.; Souza, D.E.R.; Filho, J.D.S.; Oliveira, A.B. Chemistry and antiviral activity of Arrabidaea pulchra (Bignoniaceae). Molecules 2013, 18, 9919–9932. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, A.T.; Bêdo, T.R.F.O.; Braguine, C.G.; Gonçalves, U.O.; Magalhães, L.G.; Rodrigues, V.; Gimenez, V.M.M.; Groppo, M.; Silva, M.L.A.; Cunha, W.R.; et al. Evaluation of Cuspidaria pulchra and its Isolated Compounds Against Schistosoma mansoni Adult Worms. Int. J. Biotechnol. Wellness Ind. 2012, 1, 122–127. [Google Scholar] [CrossRef]
- Leite, J.P.V.; Oliveira, A.B.; Lombardi, J.A.; Filho, J.D.S.; Chiari, E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol. Pharm. Bull. 2006, 29, 2307–2309. [Google Scholar] [CrossRef] [Green Version]
- Brandão, G.C.; Kroon, E.G.; Dos Santos, J.R.; Stehmann, J.R.; Lombardi, J.A.; Braga De Oliveira, A. Antiviral activity of Bignoniaceae species occurring in the state of Minas Gerais (Brazil): Part 1. Lett. Appl. Microbiol. 2010, 51, 469–476. [Google Scholar] [CrossRef]
- Alvarenga, T.A.; Bertanha, C.S.; de Oliveira, P.F.; Tavares, D.C.; Gimenez, V.M.M.; Silva, M.L.A.; Cunha, W.R.; Januário, A.H.; Pauletti, P.M. Lipoxygenase inhibitory activity of Cuspidaria pulchra and isolated compounds. Nat. Prod. Res. 2014, 29, 1083–1086. [Google Scholar] [CrossRef]
- Leite, J.P.V.; Lombardi, J.A.; Chiari, E.; Oliveira, A.B. Isolamento biomonitorado de uma substância tripanossomicida de Arrabidaea triplinervia (Bignoniaceae), o ácicIo ursólico. Rev. Bras. Farmacogn. 2001, 11, 77–87. [Google Scholar] [CrossRef]
- Gonzalez, B.; Suarez-Roca, H.; Bravo, A.; Salas-Auvert, R.; Avila, D. Chemical composition and biological activity of extracts from Arrabidaea bilabiata. Pharm. Biol. 2000, 38, 287–290. [Google Scholar] [CrossRef]
- Krebs, H.C.; Kemmerling, W.; Habermehl, G. Qualitative and quantitative determination of fluoroacetic acid in Arrabidea bilabiata and Palicourea marcgravii by 19 F-NMR spectroscopy. Toxicon 1994, 32, 909–913. [Google Scholar] [CrossRef]
- Jorge, M.P.; Madjarof, C.; Ruiz, A.L.T.G.; Fernandes, A.T.; Rodrigues, R.A.F.; de Oliveira Sousa, I.M.; Foglio, M.A.; de Carvalho, J.E. Evaluation of wound healing properties of Arrabidaea chica Verlot extract. J. Ethnopharmacol. 2008, 118, 361–366. [Google Scholar] [CrossRef]
- Jabour, F.F.; Seixas, J.N.; Tokarnia, C.H.; Brito, M.F.V. Variação da toxidez de Arrabidaea bilabiata (Bignoniaceae) em coelhos1. Pesqui. Veterinária Bras. 2006, 26, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Tokarnia, C.H.; Barbosa, J.D.; Oliveira, C.M.C.; Brito, M.F.; Oliveira, R.B.; Barbas, L.A.L. Aspectos epidemiológicos e clínico-patológicos comparados da intoxicação por Arrabidaea bilabiata (Bignoniaceae) em búfalos e bovinos. Pesqui. Veterinária Bras. 2004, 24, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, T.C.; Oliveira, L.I.; Caldas, S.A.; Junior, F.E.A.C.; Carvalho, M.G.; França, T.N.; Peixoto, P.V. Efeito protetor da acetamida sobre as intoxicações experimentais em ratos por monofluoroacetato de sódio e por algumas plantas brasileiras que causam morte súbita. Pesqui. Vet. Bras. 2011, 31, 938–952. [Google Scholar] [CrossRef] [Green Version]
- Da Rocha, C.Q.; Vilela, F.C.; Santa-Cecília, F.V.; Cavalcante, G.P.; Vilegas, W.; Giusti-Paiva, A.; dos Santos, M.H. Oleanane-type triterpenoid: An anti-inflammatory compound of the roots Arrabidaea brachypoda. Rev. Bras. Farmacogn. 2015, 25, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Rocha, V.P.C.; Rocha, C.Q.; Queiroz, E.F.; Marcourt, L.; Vilegas, W.; Grimaldi, G.B.; Furrer, P.; Allémann, E.; Wolfender, J.; Soares, M.B.P. Antileishmanial activity of dimeric flavonoids isolated from Arrabidaea brachypoda. Molecules 2019, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Nunes, H.L.; Tuttis, K.; Serpeloni, J.M.; Nascimento, J.R.; da Rocha, C.Q.; Silva, V.A.O.; Lengert, A.V.H.; Reis, R.M.; Cólus, I.M. Characterization of the in vitro cytotoxic effects of brachydins isolated from Fridericia platyphylla in a prostate cancer cell line. J. Toxicol. Environ. Health-Part A Curr. Issues 2020, 83, 547–558. [Google Scholar] [CrossRef]
- Salgado, C.; Morin, H.; Aquino, N.; Neff, L.; Rocha, C.Q.; Vilegas, W.; Marcourt, L.; Wolfender, J.; Jordan, O.; Queiroz, E.F.; et al. In Vitro Anti-Inflammatory Activity in Arthritic Synoviocytes of A. brachypoda Root Extracts and Its Unusual Dimeric Flavonoids. Molecules 2020, 25, 5219. [Google Scholar] [CrossRef]
- Bertanha, C.S.; Gimenez, V.M.M.; Furtado, R.A.; Tavares, D.C.; Cunha, W.R.; Silva, M.L.A.; Januario, A.H.; Borges, A.; Kawano, D.F.; Parreira, R.L.T.; et al. Isolation, in vitro and in silico Evaluation of Phenylethanoid Glycoside from Arrabidaea brachypoda as Lipoxygenase Inhibitor. J. Braz. Chem. Soc 2020, 13, 849–855. [Google Scholar] [CrossRef]
- Monteiro, F.S.; Costa, J.R.S.; Martins, L.J.A.; Rocha, C.Q.; Borges, A.C.R.; Borges, M.O.R. Hydroalcoholic extract of leaves of Arrabidaea brachypoda (Dc.) bureau present antispasmodic activity mediated through calcium influx blockage. Rev. Ciencias Farm. Basica Apl. 2020, 41, 1–13. [Google Scholar] [CrossRef]
- Rezende-Júnior, L.M.; Leal, L.M.S.A.; Alves, A.L.; Mesquita, A.B.S.; Santos, A.L.P.A.; Neto, J.S.L.; Siqueira-Júnior, J.P.; Nogueira, C.E.S.; Kaatz, G.W.; Coutinho, H.D.M.; et al. Chalcones Isolated from Arrabidaea brachypoda Flowers as Inhibitors of NorA and MepA Multidrug Efflux Pumps of Staphylococcus aureus. Antibiotics 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Franco, Y.E.M.; Lima, C.A.; Rosa, M.N.; Silva, V.A.O.; Reis, R.M.; Priolli, D.G.; Carvalho, P.O.; Nascimento, J.R.; Rocha, C.Q.; Longato, G.B. Investigation of U-251 cell death triggered by flavonoid luteolin: Towards a better understanding on its anticancer property against glioblastomas. Nat. Prod. Res. 2020, 35, 4807–4813. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, I.; Tavares, M.; Amorim, J.M.; de Barros, P.A.V.; Brandão, G.C.; Contarini, S.M.L.; Souza, S.; Melo, E.L.; de Almeida-Leite, C.M.; Martins, F.S.; et al. Chemical Characterization and Anti-inflammatory Assessment of the Hydroethanolic Extract of Fridericia chica. Rev. Bras. Farmacogn. 2020, 30, 559–567. [Google Scholar] [CrossRef]
- Do Amaral, R.R.; Santos, A.A.D.; Saravia, A.; Botas, G.; Cruz, R.A.S.; Fernandes, C.P.; Rocha, L.; Boylan, F. Biological activities of Arrabidaea chica (Bonpl.) B. Verl. leaves. Lat. Am. J. Pharm. 2012, 31, 451–455. [Google Scholar]
- Siqueira, F.C.; Leitão, D.S.T.C.; Mercadante, A.Z.; Chisté, R.C.; Lopes, A.S. Profile of phenolic compounds and carotenoids of Arrabidaea chica leaves and the in vitro singlet oxygen quenching capacity of their hydrophilic extract. Food Res. Int. 2019, 126, 108597. [Google Scholar] [CrossRef]
- Horie, T.; Tominaga, H.; Kawamura, Y.; Hada, T.; Ueda, N.; Amano, Y.; Yamamoto, S. Syntheses of 5,7,8- and 5,6,7-Trioxygenated 3-Alkyl-3′,4′-dihydroxyflavones and Their Inhibitory Activities against Arachidonate 5-Lipoxygenase. J. Med. Chem. 1991, 34, 2169–2176. [Google Scholar] [CrossRef]
- Barbosa, W.L.R.; Pinto, L.N.; Quignard, E.; Vieira, J.M.S.; Silva, J.O.C.; Albuquerque, S. Arrabidaea chica (HBK) Verlot: Phytochemical approach, antifungal and trypanocidal activities. Rev. Bras. Farmacogn. 2008, 18, 544–548. [Google Scholar] [CrossRef]
- Takemura, O.S.; Iinuma, M.; Tosa, H.; Miguel, O.G.; Moreira, E.A.; Nozawa, Y. A flavone from leaves of Arrabidaea chica F. cuprea. Phytochemistry 1995, 38, 1299–1300. [Google Scholar] [CrossRef]
- Violante, I.M.P.; Carollo, C.A.; Silva, L.I.; Oliveira, A.Q.C.; Pardinho, F.C.; Garcez, W.S.; Garcez, F.R.; Arunachalam, K.; Martins, D.T.O. Cytotoxicity and antibacterial activity of scutellarein and carajurone-enriched fraction obtained from the hydroethanolic extract of the leaves of Fridericia chica (Bonpl.) L.G. Lohmann. Nat. Prod. Res. 2020, 35, 5287–5293. [Google Scholar] [CrossRef]
- Lima, J.C.S.; de Oliveira, R.G.; Silva, V.C.; de Sousa, P.T.; Violante, I.M.P.; Macho, A.; Martins, D.T.O. Anti-inflammatory activity of 4′,6,7-trihydroxy-5-methoxyflavone from Fridericia chica (Bonpl.) L.G.Lohmann. Nat. Prod. Res. 2020, 34, 726–730. [Google Scholar] [CrossRef]
- Moragas-Tellis, C.J.; Almeida-Souza, F.; Chagas, M.S.S.; de Souza, P.V.R.; Silva-Silva, J.V.; Ramos, Y.J.; Moreira, D.L.; Calabrese, K.S.; Behrens, M.D. The influence of anthocyanidin profile on antileishmanial activity of Arrabidaea chica morphotypes. Molecules 2020, 25, 3547. [Google Scholar] [CrossRef]
- Ribeiro, A.F.C.; Telles, T.C.; Ferraz, V.P.; Souza-Fagundes, E.M.; Cassali, G.D.; Carvalho, A.T.; Melo, M.M. Effect of Arrabidaea chica extracts on the Ehrlich solid tumor development. Braz. J. Pharmacogn. 2012, 22, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.P.C.; Borrás, M.R.L.; Ferreira, L.C.L.; López-Lozano, J.L. Atividade antiinflamatória do extrato aquoso de Arrabidaea chica (Humb. & Bonpl.) B. Verl. sobre o edema induzido por venenos de serpentes amazônicas. Rev. Bras. Farmacogn. 2009, 19, 643–649. [Google Scholar] [CrossRef]
- Taffarello, D.; Jorge, M.P.; Sousa, I.M.O.; Duarte, M.C.T.; Figueira, G.M.; Queiroz, N.C.A.; Rodrigues, R.A.F.; De Carvalho, J.E.; Goes, A.L.T.R.; Foglio, M.A.; et al. Atividade de extratos de Arrabidaea chica (HUMB. & BONPL.) verlot obtidos por processos biotecnológicos sobre a proliferação de fibroblastos e células tumorais humanas. Quim. Nova 2013, 36, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, B.J.L.; Costa, K.S.; Ribeiro, J.F.A.; Silva, J.O.C.; Barbosa, W.L.R.; Carvalho, J.C.T. Liver protective activity of a hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl. (pariri). Pharmacogn. Res. 2011, 3, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.S.M.; Mendes, P.C.; Vieira, J.G.P.; Ozela, E.F.; Barbosa, W.L.R.; Júnior, J.O.C.S. Análise farmacognóstica das folhas de Arrabidaea chica (Humb. & Bonpl.) B. Verlt. Bignoniaceae. Braz. J. Pharmacogn. 2010, 20, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Paula, J.T.; Paviani, L.C.; Foglio, M.A.; Sousa, I.M.O.; Cabral, F.A. Extraction of anthocyanins from Arrabidaea chica in fixed bed using CO2 and CO2/ethanol/water mixtures as solvents. J. Supercrit. Fluids 2013, 81, 33–41. [Google Scholar] [CrossRef]
- Mafioleti, L.; Junior, I.F.S.; Colodel, E.M.; Flach, A.; Martins, D.T.O. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013, 150, 576–582. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Azevedo, M.M.B.; Chaves, F.C.M.; Alviano, C.S.; Alviano, D.S.; Vermelho, A.B. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. Biomed Res. Int. 2014, 2014, 985171. [Google Scholar] [CrossRef] [Green Version]
- Aro, A.A.; Simões, G.F.; Esquisatto, M.A.M.; Foglio, M.A.; Carvalho, J.E.; Oliveira, A.L.R.; Gomes, L.; Pimentel, E.R. Arrabidaea chica extract improves gait recovery and changes collagen content during healing of the Achilles tendon. Injury 2013, 44, 884–892. [Google Scholar] [CrossRef]
- Gemelli, T.F.; Prado, L.S.; Santos, F.S.; Souza, A.P.; Guecheva, T.N.; Henriques, J.A.P.; Ferraz, A.B.F.; Corrêa, D.S.; Dihl, R.R.; Picada, J.N. Evaluation of Safety of Arrabidaea chica Verlot (Bignoniaceae), a Plant with Healing Properties. J. Toxicol. Environ. Health-Part A Curr. Issues 2015, 78, 1170–1180. [Google Scholar] [CrossRef]
- Servat-Medina, L.; González-Gómez, A.; Reyes-Ortega, F.; Sousa, I.M.O.; Queiroz, N.C.A.; Zago, P.M.W.; Jorge, M.P.; Monteiro, K.M.; de Carvalho, J.E.; San Román, J.; et al. Chitosan–tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015, 10, 3897–3909. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, I.R.S.; Soares, A.O.; Araújo, L.M.; Da Costa, P.R.C.; Roland, I.A.; Borrás, M.R.L. Determination of Cu, Fe, Mn, and Zn in the leaves and tea of Arrabidaea chica (Humb. & Bompl.) Verl. Biol. Trace Elem. Res. 2009, 132, 239–246. [Google Scholar] [CrossRef]
- Sá, J.C.; Almeida-Souza, F.; Mondêgo-Oliveira, R.; Oliveira, I.S.S.; Lamarck, L.; Magalhães, I.F.B.; Ataídes-Lima, A.F.; Ferreira, H.S.; Abreu-Silva, A.L. Leishmanicidal, cytotoxicity and wound healing potential of Arrabidaea chica Verlot. BMC Complement. Altern. Med. 2016, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, V.C.; Longo, T.B.; Garcia, A.L.H.; Richter, M.F.; Guecheva, T.N.; Henriques, J.A.P.; Ferraz, A.B.F.; Picada, J.N. Evaluation of the mutagenicity and genotoxicity of Arrabidaea chica verlot (Bignoneaceae), an amazon plant with medicinal properties. J. Toxicol. Environ. Health-Part A Curr. Issues 2013, 76, 381–390. [Google Scholar] [CrossRef]
- Rocha, K.B.F.; Oliveira, C.N.; Azevedo, I.M.; de Macedo, R.; Medeiros, A.C. Effect of Arrabidaea chica extract against chemically induced breast cancer in animal model. Acta Cir. Bras. 2019, 34, e201901001. [Google Scholar] [CrossRef]
- Pires, A.L.R.; Westin, C.B.; Hernandez-Montelongo, J.; Sousa, I.M.O.; Foglio, M.A.; Moraes, A.M. Flexible, dense and porous chitosan and alginate membranes containing the standardized extract of Arrabidaea chica Verlot for the treatment of skin lesions. Mater. Sci. Eng. C 2020, 112, 110869. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Volpato, H.; Lazarin-Bidóia, D.; Desoti, V.C.; de Souza, R.O.; Fonseca, M.J.V.; Ueda-Nakamura, T.; Nakamura, C.V.; Silva, S.O. The extended production of UV-induced reactive oxygen species in L929 fibroblasts is attenuated by posttreatment with Arrabidaea chica through scavenging mechanisms. J. Photochem. Photobiol. B Biol. 2018, 178, 175–181. [Google Scholar] [CrossRef]
- Siraichi, J.T.G.; Pedrochi, F.; Natali, M.R.M.; Ueda-Nakamura, T.; Filho, B.P.D.; Bento, A.C.; Baesso, M.L.; Nakamura, C.V. Ultraviolet (UVB and UVA) photoprotector activity and percutaneous penetration of extracts obtained from Arrabidaea chica. Appl. Spectrosc. 2013, 67, 1179–1184. [Google Scholar] [CrossRef]
- Zago, P.M.W.; Sousa, I.M.O.; Servat-Medina, L.; Jorge, M.P.; Neto, L.G.L.; Hass, V.; Li, X.; Ruiz, A.L.T.G.; Saxena, D.; Foglio, M.A. Standardized Arrabidaea chica extract shows cytoprotective effects in zoledronic acid-treated fibroblasts and osteoblasts. Clin. Cosmet. Investig. Dent. 2020, 12, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Hay, A.E.; Cressend, D.; Reist, M.; Vivas, L.; Gupta, M.P.; Carrupt, P.A.; Hostettmann, K. Antioxidant C-glucosylxanthones from the leaves of Arrabidaea patellifera. J. Nat. Prod. 2008, 71, 1887–1890. [Google Scholar] [CrossRef] [PubMed]

| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 1 |  | A. samydoides | Leaves and stem, EtOH | Not evaluated | [1] |
| 2 |  | A. samydoides | Leaves and stem, EtOH | Not evaluated | [1] |
| 3 |  | A. samydoides and A. bilabiata | Leaves and stem, EtOH | Not evaluated | [1] |
| 4 |  | A. samydoides | Leaves and stem, EtOH | Not evaluated | [1] |
| 5a |  | A. samydoides | Leaves and stem, EtOH | Not evaluated | [1] |
| 5b |  | A. samydoides | Leaves and stem, EtOH | Not evaluated | [1] |
| 6a |  | A. samydoides and A. pulchra and A. triplinervia and A. bilabiata | Leaves and stem, EtOH | Not evaluated | [1] |
| 6b |  | A. samydoides | Leaves and stem, EtOH | Trypanocidal | [1,14,15,16] |
| 7a |  | A. samydoides | Stem, EtOH | Antioxidant | [10] |
| 7b |  | A. samydoides | Stem, EtOH | Non active | [10] |
| 7c |  | A. samydoides | Stem, EtOH | Non active | [10] |
| 7d |  | A. samydoides | Stem, EtOH | Non active | [10] |
| 7e |  | A. samydoides | Stem, EtOH | Non active | [10] |
| 7f |  | A. samydoides | Stem, EtOH | Antioxidant | [10] |
| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 8a |  | A. pulchra | Leaves, EtOH | Antiviral | [14] |
| 8b |  | A. pulchra | Aerial parts, EtOH | 15-LOX inhibitory | [18] |
| 9 |  | A. pulchra | Leaves and aerial parts EtOH | Antiviral and 15-LOX inhibitory | [14,18] |
| 10 |  | A. pulchra | Aerial parts, EtOH | 15-LOX inhibitory | [18] |
| 11 |  | A. pulchra | Aerial parts, EtOH | Non active | [15] |
| 12 |  | A. pulchra | Aerial parts, EtOH | Non active | [15] |
| 13 |  | A. pulchra | Aerial parts, EtOH | Non active | [15] |
| 14 |  | A. pulchra and A. triplinervia | Aerial parts, EtOH | Trypanocidal | [15,16] |
| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 15 |  | A. triplinervia | Leaves, EtOH | Non active | [16] |
| 16 |  | A. triplinervia | Leaves, EtOH | Non active | [16] |
| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 17 |  | A. bilabiata | Seeds and leaves, aqueous extract | Not evaluated | [21] |
| 18 |  | A. bilabiata and A. chica | Aerial parts, IsopOH | Motor activity | [20,22] |
| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 19a |  | A. brachypoda | Leaves, chloroform | Fungicide | [7] |
| 19b |  | A. brachypoda | Leaves, chloroform | Fungicide | [7] |
| 19c |  | A. brachypoda | Leaves, chloroform | Fungicide | [7] |
| 19d |  | A. brachypoda and A. chica | Leaves, chloroform | Non active | [7,34] |
| 20 |  | A. brachypoda | Roots, EtOH | Anti-inflammatory | [26] |
| 21a |  | A. brachypoda | Roots, EtOH | Not evaluated | [11] |
| 21b |  | A. brachypoda | Roots, EtOH | Not evaluated | [11] |
| 22a |  | A. brachypoda | Roots, EtOH | Antiproliferative | [11,27,28,29] |
| 22b |  | A. brachypoda | Roots, EtOH | Leishmania, Antiproliferative and arthritis | [11,27,28,29] |
| 22c |  | A. brachypoda | Roots, EtOH | Antiproliferative and arthritis | [11,27,28] |
| 22d–j |  | A. brachypoda | Roots, EtOH | Gastroprotective | [11] |
| 23a–p |  | A. brachypoda | Roots, EtOH | Trypanocidal (23e, 23k, 23n, 23o) and Leishmanicidal (23g and 230) | [12] |
| 24 |  | A. brachypoda | Aerial parts, EtOH | Inhibition of LOX | [30] |
| 25 |  | A. brachypoda and A. chica | Leaves, EtOH | Not evaluated | [31,35] |
| 26 |  | A. brachypoda and A. chica | Leaves and roots, EtOH | Antiproliferative and diuretic | [31,33,35,36] |
| 27 |  | A. brachypoda and A. chica | Leaves, EtOH | Not evaluated | [31] |
| 28 |  | A. brachypoda | Leaves, EtOH | Not evaluated | [31] |
| 29 |  | A. brachypoda | Flowers, EtOH | Non active | [32] |
| 30 |  | A. brachypoda | Flowers, EtOH | Non active | [32] |
| 31 |  | A. brachypoda | Flowers, EtOH | Non active | [32] |
| 32 |  | A. brachypoda | Flowers, EtOH | Antimicrobial | [32] |
| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 33a |  | A. chica | Leaves, Et2O | Inhibited NF-kB | [8,42] |
| 33b |  | A. chica | Leaves, Et2O | Non active | [8,40] |
| 33c |  | A. chica | Leaves, Et2O | Non active | [8,42] |
| 33d |  | A. chica | Leaves, Et2O | Non active | [8,42] |
| 34 |  | A. chica | Leaves, MeOH | Non active | [39] |
| 35 |  | A. chica | Leaves, EtOH | Not evaluated | [38] |
| 36 |  | A. chica | Leaves, EtOH | Not evaluated | [38] |
| 37 |  | A. chica | Leaves, EtOH | Not evaluated | [38] |
| 38a |  | A. chica | Leaves, EtOH | Non active | [34,36,40] |
| 38b |  | A. chica | Leaves, EtOH | Not evaluated | [34] |
| 39 |  | A. chica | Leaves, EtOH | Anti-inflammatory | [41] |
| 40a |  | A. chica | Leaves, Acetone | Not evaluated | [36] |
| 40b |  | A. chica | Leaves, Acetone | Not evaluated | [36] |
| 41a |  | A. chica | Leaves, EtOH | Not evaluated | [34] |
| 41b |  | A. chica | Leaves, EtOH | Not evaluated | [34] |
| 41c |  | A. chica | Leaves, EtOH | Not evaluated | [34] |
| N° | Compound Structure and Name | Species Name | Plant Part, Solvent | Biological Effect | Ref. |
|---|---|---|---|---|---|
| 42a |  | A. patellifera | Leaves, MeOH | Antioxidant and Plasmodium falciparum | [62] |
| 42b |  | A. patellifera | Leaves, MeOH | Antioxidant and Plasmodium falciparum | [62] |
| 42c |  | A. patellifera | Leaves, MeOH | Antioxidant and Plasmodium falciparum | [62] |
| 42d |  | A. patellifera | Leaves, MeOH | Antioxidant and Plasmodium falciparum | [62] |
| 42e |  | A. patellifera | Leaves, MeOH | Antioxidant | [62] |
| 42f |  | A. patellifera | Leaves, MeOH | Antioxidant | [62] |
| 42g |  | A. patellifera | Leaves, MeOH | Antioxidant | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, J.R.; de Jesus Alves Miranda, A.; Vieira, F.C.; Rodrigues, C.D.P.; Vasconcelos, L.N.; Filho, J.L.P.; Lopes, A.C.C.B.; Tangerina, M.M.P.; Vilegas, W.; da Rocha, C.Q. A Review of the Phytochemistry and Pharmacological Properties of the Genus Arrabidaea. Pharmaceuticals 2022, 15, 658. https://doi.org/10.3390/ph15060658
do Nascimento JR, de Jesus Alves Miranda A, Vieira FC, Rodrigues CDP, Vasconcelos LN, Filho JLP, Lopes ACCB, Tangerina MMP, Vilegas W, da Rocha CQ. A Review of the Phytochemistry and Pharmacological Properties of the Genus Arrabidaea. Pharmaceuticals. 2022; 15(6):658. https://doi.org/10.3390/ph15060658
Chicago/Turabian Styledo Nascimento, Jessyane Rodrigues, Amanda de Jesus Alves Miranda, Felipe Costa Vieira, Carla Daniele Pinheiro Rodrigues, Luna Nascimento Vasconcelos, José Lima Pereira Filho, Auxiliadora Cristina Corrêa Barata Lopes, Marcelo Marucci Pereira Tangerina, Wagner Vilegas, and Cláudia Quintino da Rocha. 2022. "A Review of the Phytochemistry and Pharmacological Properties of the Genus Arrabidaea" Pharmaceuticals 15, no. 6: 658. https://doi.org/10.3390/ph15060658